

▌ Overview of Synthetic Antibody Libraries
In the quest for superior therapeutic and diagnostic reagents, synthetic antibody libraries represent a paradigm shift from natural immune repertoires. Unlike libraries derived from immunized animals or human donors, synthetic libraries are built de novo through rational design and gene synthesis. This approach offers unparalleled control over the final product's properties.
A synthetic library is constructed on one or more optimized, stable antibody frameworks. Diversity is intentionally introduced into the Complementarity-Determining Regions (CDRs), the loops responsible for antigen binding, using defined genetic algorithms. This allows for the creation of vast, fully humanized (or other species) repertoires that are not limited by the natural immune system's biases, tolerance mechanisms, or the need for immunization. Synthetic VHH (Variable Heavy-chain of Heavy-chain antibody, or Nanobody) libraries leverage these principles to create compact, stable, and highly diverse single-domain antibody repertoires ideal for targeting complex epitopes.
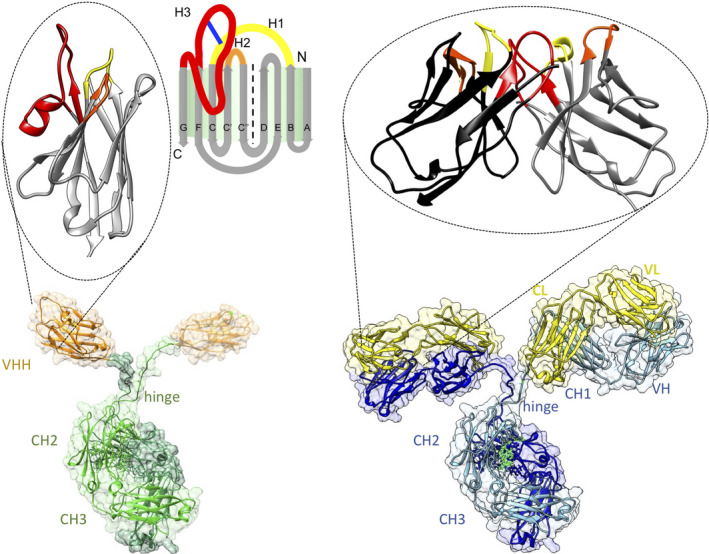
Figure1.Architecture of homodimeric heavy‐chain antibodies (bottom left) with the Ag‐binding single‐domain VHH, enlarged on top; and classical heterotetrameric antibodies (bottom right) with the Ag‐binding variable fragments (comprising VH and VL domains) enlarged on top. (Muyldermans S. A guide to: generation and design of nanobodies.FEBS J. 2021;288(7):2084-2102.)
▌ What is Phage Display Technology?
Phage display is a powerful in vitro evolution platform that enables the screening of vast protein libraries for desired functions. It works by physically linking a protein's phenotype (its function, e.g., binding) to its genotype (its DNA sequence). In this system, the gene encoding a protein of interest—in this case, a VHH—is fused to a gene encoding a coat protein of a bacteriophage (a virus that infects bacteria).
When the engineered phage infects E. coli, it replicates and assembles new viral particles. Crucially, the VHH protein is displayed on the phage's surface, while the DNA encoding it is packaged inside the same particle. To find a VHH that binds a specific target, a diverse library of these VHH-displaying phages is exposed to the immobilized target. Non-binders are washed away, and the bound phages are eluted and amplified in bacteria. Repeating this "biopanning" cycle 3-5 times enriches the population for high-affinity, specific binders, whose genes are easily retrieved from the phage for downstream use.
▌Key Elements in Synthetic VHH Library Design
The quality and utility of a synthetic VHH library are determined at the design stage. Our expertise focuses on four critical pillars:
● Diversity & Size: True functional diversity is paramount. We design libraries with theoretical diversities exceeding 10^10 unique members. Diversity is strategically concentrated in the CDR loops, especially CDR3, which is the primary determinant of specificity in VHHs. We use trinucleotide mutagenesis or other advanced techniques to ensure balanced amino acid representation and avoid stop codons.
● Framework Selection & Humanization: We employ well-characterized, highly stable VHH frameworks with proven expression yields in microbial systems. For therapeutic applications, frameworks can be chosen or engineered to be highly humanized, minimizing immunogenicity risk in patients. The framework provides the stable scaffold upon which diverse CDRs are mounted.
● Stability & Developability by Design: Unlike naive design, we incorporate developability criteria from the outset. Our design algorithms can bias against sequences prone to aggregation, with unusual hydrophobicity, or with potential post-translational modification sites. This pre-selection increases the fraction of library members that are well-expressed, stable, and suitable for development.
● CDR Length & Composition Design: We carefully design the variability in CDR lengths and amino acid composition. CDR-H3 lengths can be varied to target different epitope topologies (longer for concave epitopes). The chemical diversity in the CDRs is tailored to promote interactions such as hydrogen bonding, salt bridges, and hydrophobic contacts.
▌Library Construction Workflow
Nebulabio’s construction process is a meticulous, multi-stage operation ensuring the final library matches the designed specifications with high fidelity.
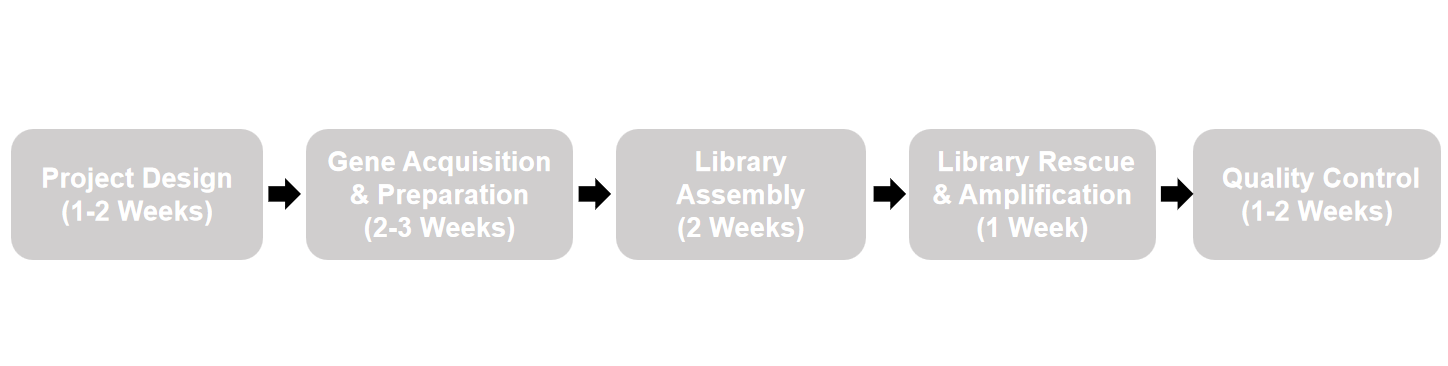
Step 1: In Silico Design & Gene Synthesis
Our proprietary bioinformatics pipeline generates millions of unique VHH sequences based on the design parameters. From this pool, a diverse subset representing the full sequence space is selected. The corresponding DNA sequences are synthesized as oligonucleotide pools or gene fragments with optimized codon usage for high-yield expression in E. coli.
Step 2: Assembly & Cloning
The synthesized gene fragments are assembled into full-length VHH genes via PCR-based methods. These genes are then cloned in bulk into our proprietary phage display vector. This vector is engineered for optimal VHH expression as a fusion to the M13 phage pIII protein and contains all necessary elements for phage production and antibiotic selection.
Step 3: High-Efficiency Transformation
The ligated plasmid library is introduced into electrocompetent E. coli cells via high-voltage electroporation. This step is critical for achieving the actual library size that matches the theoretical design. We use optimized conditions to maximize transformation efficiency, resulting in a primary bacterial library containing billions of independent clones.
Step 4: Quality Control & Validation
We perform rigorous QC to validate the library before delivery:
● Library Titer: Determination of the actual colony-forming units (CFU) to confirm library size.
● Insert Rate & Sequencing: PCR analysis of random colonies to verify the presence and correct size of the VHH insert. Next-Generation Sequencing (NGS) of the library pool is performed to analyze the realized diversity, CDR3 length distribution, and amino acid frequency, comparing it to the original design.
● Functional Spot Check: A small-scale test panning against a standard antigen may be performed to confirm the library's functionality and ability to yield binders.
Step 5: Delivery
The final deliverable includes the primary bacterial glycerol stock, the rescued phage display library, and a comprehensive QC dossier with NGS data and analytical reports.
▌ Applications of Synthetic VHH Libraries
Synthetic VHH libraries are versatile tools powering innovation across biotechnology:
● Therapeutic Drug Discovery: Discovery of fully human, high-affinity VHH leads against challenging targets like GPCRs, ion channels, and intracellular proteins. Their small size allows for engineering into multi-specific formats or crossing the blood-brain barrier.
● Cell & Gene Therapy: Engineering VHH-based binding domains for next-generation CAR-T cells, where their small size and stability can lead to better receptor performance.
● Diagnostic Imaging: VHHs are ideal for in vivo molecular imaging (PET, SPECT) due to rapid tissue penetration and blood clearance, providing high-contrast images.
● Antivirals & Neutralizing Agents: Rapid discovery of VHHs that block viral entry or neutralize toxins, leveraging their ability to bind cryptic, conserved epitopes.
● Agricultural & Environmental Biosensors: Developing stable, reliable binding reagents for field-deployable detection systems.
● Research Reagents & Intrabodies: Creating highly specific inhibitors or stabilizers of protein function for basic research, including intracellular use (intrabodies).
▌ Challenges and Considerations
While powerful, synthetic library construction and use involve specific considerations:
● Design Complexity: Moving from a naive to a rationally designed library requires sophisticated bioinformatics and a deep understanding of antibody structure-function relationships. Poor design leads to non-functional libraries.
● Cost & Time: The gene synthesis and large-scale cloning required are more resource-intensive upfront than isolating genes from natural sources.
● Risk of "Over-Design": Excessive bias for stability or humanization might inadvertently constrain the natural structural diversity needed to bind some targets. Finding the right balance is key.
● Screening Strategy: Synthetic libraries, while diverse, lack the in vivo affinity maturation of immune libraries. Therefore, they may initially yield binders with moderate affinity, often requiring a subsequent in vitro affinity maturation step to reach therapeutic-grade potency.
● Framework Limitations: The choice of a single or limited set of frameworks, while beneficial for predictability, means all selected binders share these backbone properties, which may not be optimal for every application.
Partner with Nebulabio to leverage our expertise in cutting-edge synthetic biology and antibody engineering. We will work with you to design and build a custom synthetic VHH library that serves as a powerful, enduring resource for your discovery pipeline, tailored to your specific target class and application goals.